
Product Specification
Lanlang ® TY CH560 is an incredible chelating resin that boasts a special macroporous structure and features weakly basic Bis-Picolylamine on the styrene and DVB copolymer.
Ore bodies around the world are declining in grade, whilst increasing in complexity. The level of impurities relative to the valuable metal is steadily increasing, posing new challenges to existing operations.
Ion exchange is widely used in the hydrometallurgical industry for both primary recovery of metals and the removal of impurities. The superior selectivity of ion exchange resins makes them exceptionally suitable for the removal of target impurities to very low levels, thereby saving operating costs, increasing the value of the final product and significantly improving revenue.The ion exchange process in these applications is very simple, using standard ion exchange equipment and acid regeneration. The process can be fully automated, requiring minimal operator interference and supervision.
The industrial production of copper consists of two steps. The primary step is a pyrometallurgical process, producing blister copper with a purity of approximately 99% copper. This metal is cast into anodes. During the subsequent refining step, the anodes are subjected to electrolysis to produce high purity copper (> 99.99%).In the copper electrolysis process, the impure copper anodes and cathodes (made of stainless steel or high-purity copper) are immersed in an electrolysis tank filled with copper sulphate liquor. Direct current is applied, causing dissolution of Cu2+ at the anodes with the release of two electrons. At the cathode, the opposite reaction takes place, i.e. the copper(II) ion receives two electrons and deposits as pure copper metal.
The chemical properties of nickel and cobalt are quite similar, making it difficult to remove small amounts of nickel from cobalt sulfate liquor. Various methods exist, with varying degrees of success. Precipitation typically result in high levels of co-precipitated cobalt and subsequent recycling of the cobalt within the process. Solvent extraction introduces the risk of fire and organic contamination, as well as the introduction of undesirable sodium via the pH control with sodium hydroxide.
Basic Features
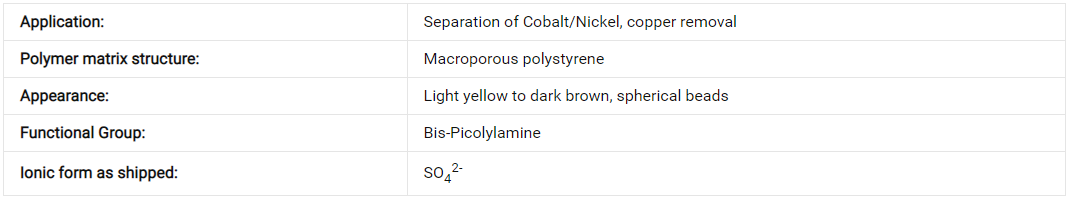
Certification

Hot Tags: ion exchange resin for nickel and copper removal, China ion exchange resin for nickel and copper removal manufacturers, suppliers, factory, palladium extracted Resin, Wet metallurgy Ion Exchange Resin, Resin for recovery rhenium, Amberlite equivalent ion exchange resin, Ion Exchange Resin for Nickel And Copper Removal, Ion Exchange Resin for adsorbed metal ions


